Njengoba inezakhiwo ezikhethekile zemvelo namakhemikhali, i-kaolin iwumthombo wamaminerali ongeyona insimbi obaluleke kakhulu kuma-ceramics, ukwenza amaphepha, irabha, amapulasitiki, ama-refractories, ukuhluzwa kwe-petroleum kanye neminye imikhakha yezobuchwepheshe yemboni nezolimo kanye nekazwelonke. Ubumhlophe be-kaolin buyinkomba ebalulekile yokusetshenziswa kwayo.

Izinto ezithinta ubumhlophe be-kaolin
I-Kaolin iwuhlobo lobumba olusanhlamvu olucolekile noma idwala lobumba ikakhulukazi elakhiwe ngamaminerali e-kaolinite. Ifomula yayo yamakhemikhali eyikristalu ithi 2SiO2 · Al2O3 · 2H2O. Inani elincane lamaminerali angewona ubumba yi-quartz, i-feldspar, amaminerali ensimbi, i-titanium, i-aluminium hydroxide nama-oxides, i-organic matter, njll.
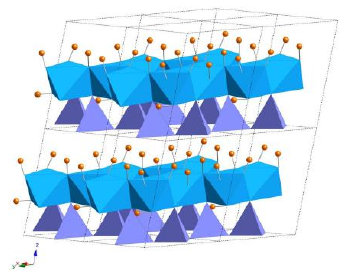
Isakhiwo se-crystalline se-kaolin
Ngokwesimo kanye nemvelo yokungcola ku-kaolin, ukungcola okubangela ukuncipha kobumhlophe be-kaolin kungahlukaniswa izigaba ezintathu: i-organic carbon; Izakhi ze-pigment, ezifana ne-Fe, Ti, V, Cr, Cu, Mn, njll; Amaminerali amnyama, njenge-biotite, i-chlorite, njll. Ngokuvamile, okuqukethwe kwe-V, Cr, Cu, Mn nezinye izakhi ku-kaolin kuncane, okunomthelela omncane ebumhlophe. Ukwakheka kwamaminerali kanye nokuqukethwe kwe-iron ne-titanium yizinto eziyinhloko ezithinta ubumhlophe be-kaolin. Ukuba khona kwazo ngeke kuthinte kuphela ubumhlophe bemvelo be-kaolin, kodwa futhi kuthinte ubumhlophe bayo obubaliwe. Ikakhulukazi, ukuba khona kwe-iron oxide kunomthelela omubi embala wobumba futhi kunciphisa ukukhanya kwawo nokumelana nomlilo. Futhi noma inani le-oxide, i-hydroxide ne-hydrated oxide ye-iron oxide ingu-0.4%, kwanele ukunikeza inzika yobumba ibomvu ibe umbala ophuzi. Lawa ma-oxide ensimbi nama-hydroxides angaba i-hematite (obomvu), i-maghemite (obomvu-nsundu), i-goethite (ophuzi onsundu), i-limonite (iwolintshi), i-hydrated iron oxide (okubomvu okunsundu), njll. Kungashiwo ukuthi ukususwa kokungcola kwensimbi. ku-kaolin idlala indima ebaluleke kakhulu ekusetshenzisweni okungcono kwe-kaolin.
Isimo sokuvela kwesici sensimbi
Isimo sokuvela kwe-iron ku-kaolin yisici esiyinhloko esinquma indlela yokukhishwa kwensimbi. Inani elikhulu lezifundo likholelwa ukuthi i-crystalline iron esesimweni sezinhlayiya ezinhle ixutshwe ku-kaolin, kuyilapho i-amorphous iron imbozwe phezu kwezinhlayiya ezinhle ze-kaolin. Njengamanje, isimo sokuvela kwensimbi ku-kaolin ihlukaniswe yaba izinhlobo ezimbili ekhaya naphesheya: eyodwa iku-kaolinite kanye namaminerali asesekeli (njenge-mica, i-titanium dioxide kanye ne-illite), ebizwa ngokuthi insimbi yesakhiwo; Enye isesimweni samaminerali ensimbi azimele, abizwa nge-iron yamahhala (okuhlanganisa i-surface iron, i-fine-grained crystalline iron ne-amorphous iron).

I-iron ekhishwe ukukhishwa kwensimbi kanye nokumhlophe kwe-kaolin iyinsimbi yamahhala, ikakhulukazi ehlanganisa i-magnetite, i-hematite, i-limonite, i-siderite, i-pyrite, i-ilmenite, i-jarosite namanye amaminerali; Insimbi eningi ikhona ngendlela ye-colloidal limonite ehlakazekile kakhulu, futhi inani elincane ngesimo se-goethite eyisiyingi, e-acicular nengavamile kanye ne-hematite.
Ukususwa kwe-ayoni kanye nendlela yokwenza mhlophe ye-kaolin
Ukuhlukaniswa kwamanzi
Le ndlela isetshenziswa kakhulu ukususa amaminerali abulalayo njenge-quartz, i-feldspar ne-mica, nokungcola okuqinile okufana nemfucumfucu yamadwala, kanye namanye amaminerali ensimbi ne-titanium. Amaminerali angcolile anokuminyana okufanayo kanye nokuncibilika ku-kaolin awakwazi ukususwa, futhi ukuthuthukiswa kobumhlophe akubonakali kahle, okulungele ukuhlomula nokuba mhlophe kwensimbi ye-kaolin esezingeni eliphezulu uma kuqhathaniswa.
Ukuhlukaniswa kwamagnetic
Ukungcola kwamaminerali okusansimbi ku-kaolin kuvame ukuba nguzibuthe obuthakathaka. Njengamanje, indlela yokuhlukanisa ngozibuthe eqinile ene-gradient ephezulu isetshenziswa kakhulu, noma amaminerali abuthaka kazibuthe aguqulwa abe yi-iron oxide enamandla kazibuthe ngemva kokugazinga, bese esuswa ngendlela evamile yokuhlukanisa kazibuthe.

Iringithoni eqondile yesihlukanisi esinegrediyenti kazibuthe

Isihlukanisi esinozibuthe esine-gradient ephezulu se-electromagnetic slurry

Izinga lokushisa eliphansi le-superconducting separator kazibuthe
Indlela ye-Flotation
Indlela yokuntanta iye yasetshenziswa ekwelapheni i-kaolin ephuma emalini eyisisekelo neyesibili. Enqubweni yokuntanta, izinhlayiya ze-kaolinite ne-mica ziyahlukaniswa, futhi imikhiqizo ehlanjululwe iyizinto ezimbalwa ezifanelekile zebanga lezimboni. Ukuhlukaniswa kwe-flotation okukhethiwe kwe-kaolinite ne-feldspar kuvame ukuqhutshwa ku-slurry nge-pH elawulwayo.
Indlela yokunciphisa
Indlela yokunciphisa iwukusebenzisa i-ejenti yokunciphisa ukunciphisa ukungcola kwensimbi (okufana ne-hematite ne-limonite) esimweni se-kaolin e-trivalent kuya kuma-ion e-iron bivalent ancibilikayo, asuswa ngokuhlungwa nokuwashwa. Ukususwa kokungcola kwe-Fe3+ ku-kaolin yezimboni kuvame ukufezwa ngokuhlanganisa ubuchwepheshe bomzimba (ukuhlukaniswa kazibuthe, i-flocculation ekhethiwe) kanye nokwelashwa kwamakhemikhali ngaphansi kwezimo ezine-asidi noma ezinciphisayo.
I-Sodium hydrosulfite (Na2S2O4), eyaziwa nangokuthi i-sodium hydrosulfite, iyasebenza ekwehliseni nasekukhipheni insimbi ku-kaolin, futhi okwamanje isetshenziswa embonini ye-kaolin. Kodwa-ke, le ndlela kufanele yenziwe ngaphansi kwezimo eziqinile ze-asidi (pH<3), okuholela ezindlekweni zokusebenza eziphezulu kanye nomthelela wemvelo. Ngaphezu kwalokho, izakhiwo zamakhemikhali ze-sodium hydrosulfite azinzile, zidinga ukugcinwa okukhethekile nokubizayo kanye nezinhlelo zokuhamba.
I-Thiourea dioxide: (NH2) 2CSO2, TD) iyi-ejenti yokunciphisa enamandla, enezinzuzo zekhono eliqinile lokunciphisa, ubungane bemvelo, izinga eliphansi lokubola, ukuphepha kanye nezindleko eziphansi zokukhiqiza inqwaba. I-Fe3+ engancibiliki ku-kaolin ingancishiswa ibe yi-Fe2+ nge-TD.
Kamuva, ubumhlophe be-kaolin bungandiswa ngemva kokuhlunga nokugeza. I-TD izinzile kakhulu ekamelweni lokushisa nezimo ezingathathi hlangothi. Amandla okunciphisa aqinile e-TD angatholakala kuphela ngaphansi kwezimo ze-alkalinity eqinile (pH>10) noma ukushisa (T>70 ° C), okuholela ezindlekweni eziphezulu zokusebenza nobunzima.
Indlela ye-oxidation
Ukwelashwa kwe-oxidation kuhlanganisa ukusetshenziswa kwe-ozone, i-hydrogen peroxide, i-potassium permanganate ne-sodium hypochlorite ukususa ungqimba lwekhabhoni ekhangisiwe ukuze kuthuthukiswe ubumhlophe. I-kaolin endaweni ejulile ngaphansi komthwalo omningi impunga, kanti i-iron ku-kaolin isesimweni sokuncipha. Sebenzisa ama-oxidizing agents aqinile njenge-ozone noma i-sodium hypochlorite ukuze ukhiphe i-FeS2 engancibiliki ku-pyrite ibe yi-Fe2+ encibilikayo, bese ugeza ukuze ususe i-Fe2+ ohlelweni.
Indlela yokukhipha i-Acid
Indlela yokukhipha i-asidi iwukuguqula ukungcola kwensimbi engancibiliki ku-kaolin kube izinto ezincibilikayo ezixazululweni ezine-asidi (i-hydrochloric acid, i-sulfuric acid, i-oxalic acid, njll.), ngaleyo ndlela ibone ukuhlukana ne-kaolin. Uma kuqhathaniswa namanye ama-asidi e-organic, i-oxalic acid ibhekwa njengethembisa kakhulu ngenxa yamandla ayo e-asidi, impahla enhle yokuhlanganisa kanye nekhono eliphezulu lokunciphisa. Nge-oxalic acid, insimbi encibilikisiwe ingancibilika esixazululweni se-leaching ngendlela ye-oxalate eyi-ferrous, futhi ingaqhutshekiselwa phambili ukuze yakhe i-hematite emsulwa ngokubala. I-Oxalic acid ingatholakala ngokushibhile kwezinye izinqubo zezimboni, futhi esigabeni sokudubula sokwenziwa kwe-ceramic, noma iyiphi i-oxalate esele ezintweni ezilashwayo izobola ibe yi-carbon dioxide. Abacwaningi abaningi baye bahlola imiphumela yokuncibilikisa i-iron oxide nge-oxalic acid.
Indlela yokubala izinga lokushisa eliphezulu
Ukubala kuyinqubo yokukhiqiza imikhiqizo ye-kaolin yebanga elikhethekile. Ngokwezinga lokushisa lokwelapha, amabanga amabili ahlukene e-kaolin e-calcined akhiqizwa. Ukubalwa ebangeni lokushisa elingu-650-700 ℃ kususa iqembu le-hydroxyl lesakhiwo, futhi umhwamuko wamanzi aphunyukayo uthuthukisa ukunwebeka nokufiphala kwe-kaolin, okuyisibaluli esifanelekile sokufakwa kwento yokumbozwa kwephepha. Ngaphezu kwalokho, ngokushisa i-kaolin ku-1000-1050 ℃, ayikwazi nje ukwandisa ukubola, kodwa futhi ithole ubumhlophe obungama-92-95%.
I-Chlorination calcination
Insimbi ne-titanium kwakhishwa kumaminerali obumba, ikakhulukazi i-kaolin nge-chlorination, futhi kwatholakala imiphumela emihle. Enqubweni ye-chlorination nokubala, ekushiseni okuphezulu (700 ℃ - 1000 ℃), i-kaolinite iye yathola i-dehydroxylation ukuze yakhe i-metakaolinite, futhi ekushiseni okuphezulu, izigaba ze-spinel ne-mullite zenziwa. Lezi zinguquko zandisa i-hydrophobicity, ubulukhuni kanye nobukhulu bezinhlayiya ngokusebenzisa i-sintering. Amaminerali aphathwa ngale ndlela angasetshenziswa ezimbonini eziningi, njengephepha, i-PVC, irabha, amapulasitiki, izinto zokunamathisela, ukupholisha nokuxubha amazinyo. I-hydrophobicity ephezulu yenza lawa maminerali ahambisane kakhulu nezinhlelo ze-organic.
Indlela ye-Microbiological
Ubuchwepheshe bokuhlanzwa kwe-microbial yamaminerali buyisifundo esisha sokucutshungulwa kwezimbiwa, okuhlanganisa ubuchwepheshe be-microbial leaching kanye nobuchwepheshe be-microbial flotation. Ubuchwepheshe be-microbial leaching of minerals ubuchwepheshe bokukhipha obusebenzisa ukuxhumana okujulile phakathi kwamagciwane amancane namaminerali ukucekela phansi i-crystal lattice yamaminerali futhi kuncibilikise izakhi eziwusizo. I-pyrite ene-oxidized namanye ama-sulfide ores aqukethwe ku-kaolin angahlanzwa ngobuchwepheshe bokukhipha ama-microbial. Ama-microorganisms asetshenziswa kakhulu afaka i-Thiobacillus ferrooxidans kanye namagciwane anciphisa i-Fe. Indlela ye-microbiological inezindleko eziphansi kanye nokungcoliswa kwemvelo okuphansi, okungeke kuthinte izici ezibonakalayo namakhemikhali e-kaolin. Kuyindlela entsha yokuhlanza kanye nokwenza mhlophe ngamathemba okuthuthukiswa kwamaminerali e-kaolin.
Isifinyezo
Ukukhishwa kwensimbi kanye nokwelashwa kokwenziwa mhlophe kwe-kaolin kudinga ukukhetha indlela engcono kakhulu ngokuya ngezimbangela ezihlukene zemibala kanye nezinjongo ezihlukene zokusetshenziswa, ukuthuthukisa ukusebenza okugcwele okumhlophe kwamaminerali e-kaolin, futhi kuyenze ibe nenani eliphezulu lokusetshenziswa kanye nenani lezomnotho. Umkhuba wokuthuthuka wesikhathi esizayo kufanele kube ukuhlanganisa izici zendlela yamakhemikhali, indlela engokomzimba kanye nendlela ye-microbiological organically, ukuze zinikeze umdlalo ogcwele ezinzuzweni zazo futhi kunqandwe ukungalungi kanye nokushiyeka kwazo, ukuze kuzuzwe umphumela ongcono wokuba mhlophe. Ngasikhathi sinye, kuyadingeka futhi ukuqhubeka nokufunda indlela entsha yezindlela ezahlukahlukene zokususa ukungcola futhi kuthuthukiswe inqubo yokwenza ukukhishwa kwe-iron nokuba mhlophe kwe-kaolin kuthuthukiswe ekuqondeni kwekhabhoni eluhlaza, ephumelelayo nephansi.
Isikhathi sokuthumela: Mar-02-2023

